If You Know the Grams of Formula How Do You Find Moles
Chapter iii. Limerick of Substances and Solutions
3.1 Formula Mass and the Mole Concept
Learning Objectives
By the end of this section, you lot will exist able to:
- Calculate formula masses for covalent and ionic compounds
- Define the amount unit mole and the related quantity Avogadro's number
Explain the relation between mass, moles, and numbers of atoms or molecules, and perform calculations deriving these quantities from one another
We tin can argue that modern chemical science began when scientists started exploring the quantitative equally well as the qualitative aspects of chemistry. For example, Dalton's diminutive theory was an attempt to explain the results of measurements that immune him to summate the relative masses of elements combined in various compounds. Understanding the relationship between the masses of atoms and the chemical formulas of compounds allows us to quantitatively describe the composition of substances.
Formula Mass
In an earlier affiliate, we described the development of the diminutive mass unit, the concept of average atomic masses, and the utilize of chemical formulas to represent the elemental makeup of substances. These ideas tin can be extended to calculate the formula mass of a substance by summing the average atomic masses of all the atoms represented in the substance's formula.
Formula Mass for Covalent Substances
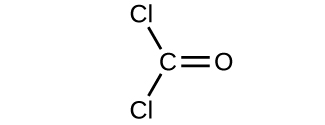
For covalent substances, the formula represents the numbers and types of atoms composing a single molecule of the substance; therefore, the formula mass may be correctly referred to every bit a molecular mass. Consider chloroform (CHClthree), a covalent chemical compound once used as a surgical anesthetic and now primarily used in the production of the "anti-stick" polymer, Teflon. The molecular formula of chloroform indicates that a single molecule contains 1 carbon atom, one hydrogen atom, and three chlorine atoms. The average molecular mass of a chloroform molecule is therefore equal to the sum of the average atomic masses of these atoms. Figure 1 outlines the calculations used to derive the molecular mass of chloroform, which is 119.37 amu.
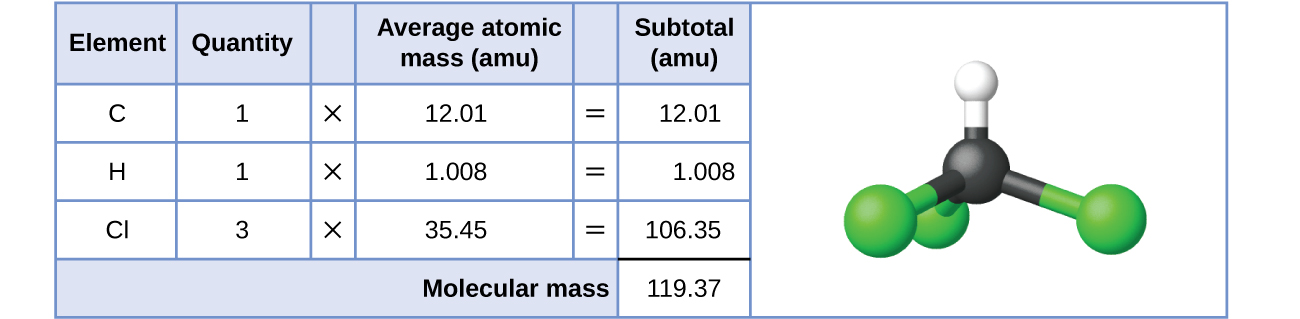
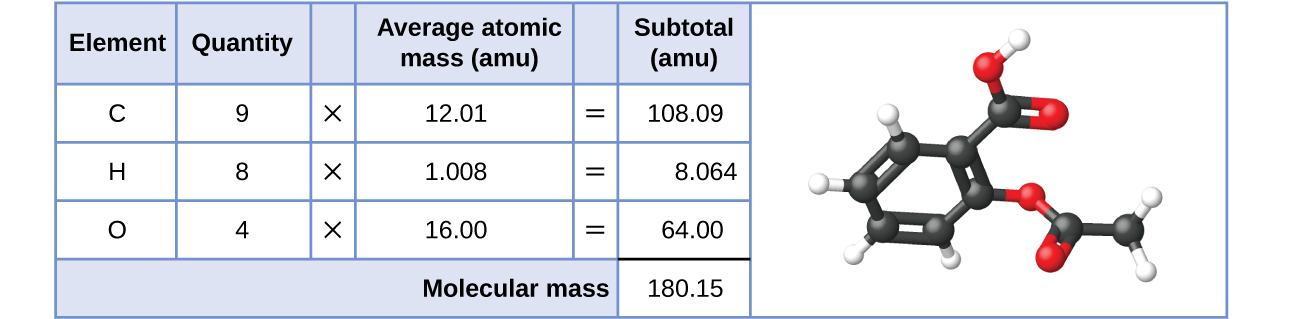
Likewise, the molecular mass of an aspirin molecule, C9H8O4, is the sum of the diminutive masses of nine carbon atoms, 8 hydrogen atoms, and four oxygen atoms, which amounts to 180.15 amu (Figure 2).
Example ane
Computing Molecular Mass for a Covalent Chemical compound
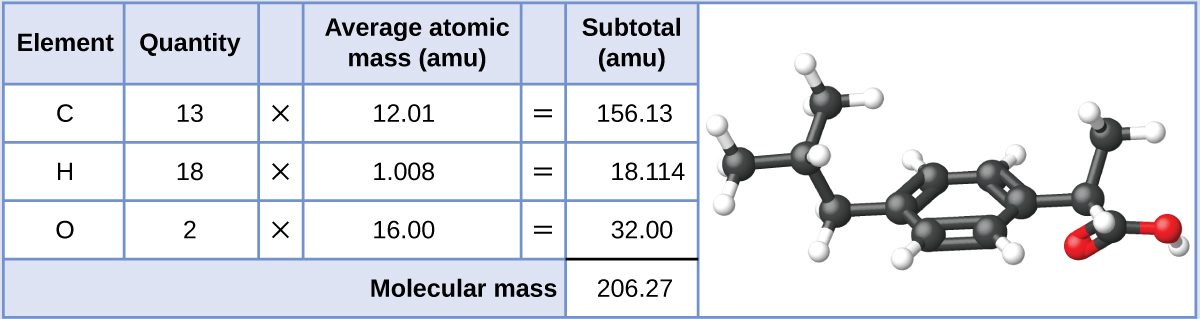
Ibuprofen, C13H18O2, is a covalent compound and the active ingredient in several pop nonprescription hurting medications, such as Advil and Motrin. What is the molecular mass (amu) for this chemical compound?
Solution
Molecules of this compound are comprised of 13 carbon atoms, 18 hydrogen atoms, and 2 oxygen atoms. Post-obit the approach described above, the average molecular mass for this chemical compound is therefore:
Check Your Learning
Acetaminophen, C8HixNO2, is a covalent compound and the active ingredient in several popular nonprescription pain medications, such as Tylenol. What is the molecular mass (amu) for this chemical compound?
Formula Mass for Ionic Compounds
Ionic compounds are composed of discrete cations and anions combined in ratios to yield electrically neutral bulk thing. The formula mass for an ionic chemical compound is calculated in the same style as the formula mass for covalent compounds: by summing the average atomic masses of all the atoms in the compound'southward formula. Keep in mind, however, that the formula for an ionic chemical compound does not correspond the composition of a discrete molecule, and then it may not correctly be referred to every bit the "molecular mass."
Every bit an example, consider sodium chloride, NaCl, the chemical name for mutual table salt. Sodium chloride is an ionic compound equanimous of sodium cations, Na+, and chloride anions, Cl−, combined in a one:1 ratio. The formula mass for this compound is computed as 58.44 amu (run across Figure three).
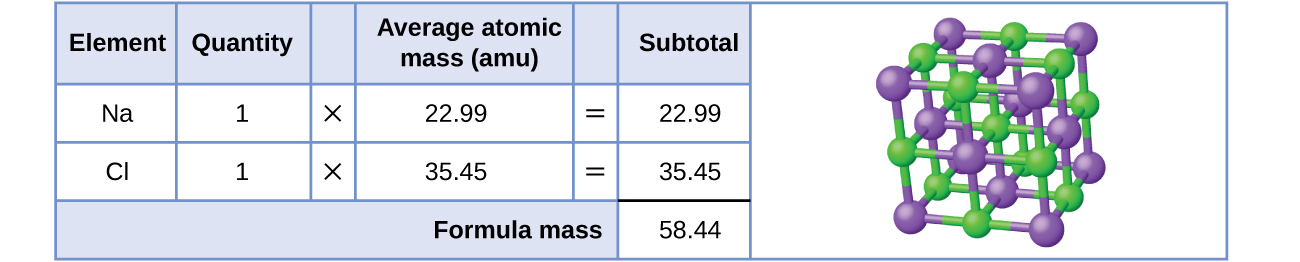
Annotation that the average masses of neutral sodium and chlorine atoms were used in this computation, rather than the masses for sodium cations and chlorine anions. This approach is perfectly adequate when computing the formula mass of an ionic compound. Fifty-fifty though a sodium cation has a slightly smaller mass than a sodium atom (since it is missing an electron), this difference will be commencement by the fact that a chloride anion is slightly more than massive than a chloride atom (due to the extra electron). Moreover, the mass of an electron is negligibly minor with respect to the mass of a typical atom. Even when computing the mass of an isolated ion, the missing or boosted electrons can generally be ignored, since their contribution to the overall mass is negligible, reflected only in the nonsignificant digits that will be lost when the computed mass is properly rounded. The few exceptions to this guideline are very light ions derived from elements with precisely known diminutive masses.
Example 2
Computing Formula Mass for an Ionic Compound
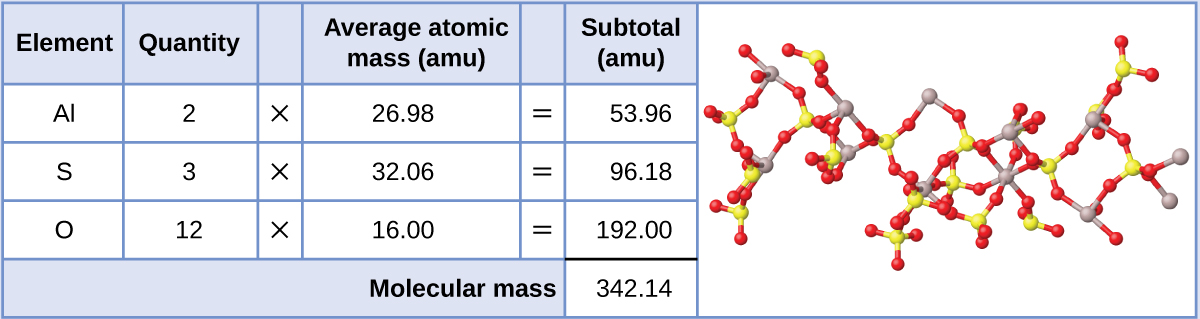
Aluminum sulfate, Al2(So4)3, is an ionic compound that is used in the manufacture of newspaper and in various water purification processes. What is the formula mass (amu) of this chemical compound?
Solution
The formula for this compound indicates information technology contains Al3+ and theniv 2− ions combined in a 2:3 ratio. For purposes of calculating a formula mass, information technology is helpful to rewrite the formula in the simpler format, Al2SthreeO12. Following the approach outlined above, the formula mass for this compound is calculated as follows:
Cheque Your Learning
Calcium phosphate, Ca3(PO4)2, is an ionic compound and a common anti-caking agent added to nutrient products. What is the formula mass (amu) of calcium phosphate?
The Mole
The identity of a substance is defined not only past the types of atoms or ions it contains, but past the quantity of each type of cantlet or ion. For example, h2o, HiiO, and hydrogen peroxide, H2Otwo, are alike in that their respective molecules are composed of hydrogen and oxygen atoms. However, because a hydrogen peroxide molecule contains 2 oxygen atoms, as opposed to the water molecule, which has only one, the two substances exhibit very unlike properties. Today, nosotros possess sophisticated instruments that allow the straight measurement of these defining microscopic traits; nonetheless, the same traits were originally derived from the measurement of macroscopic backdrop (the masses and volumes of bulk quantities of thing) using relatively uncomplicated tools (balances and volumetric glassware). This experimental approach required the introduction of a new unit for amount of substances, the mole, which remains indispensable in modern chemical science.
The mole is an amount unit similar to familiar units similar pair, dozen, gross, etc. It provides a specific measure of the number of atoms or molecules in a bulk sample of matter. A mole is defined equally the amount of substance containing the same number of detached entities (such as atoms, molecules, and ions) as the number of atoms in a sample of pure 12C weighing exactly 12 one thousand. One Latin connotation for the word "mole" is "large mass" or "bulk," which is consistent with its employ as the name for this unit. The mole provides a link betwixt an easily measured macroscopic property, bulk mass, and an extremely important fundamental property, number of atoms, molecules, and so forth.
The number of entities composing a mole has been experimentally determined to be 6.02214179 × 1023, a fundamental constant named Avogadro's number (NA ) or the Avogadro constant in honor of Italian scientist Amedeo Avogadro. This constant is properly reported with an explicit unit of "per mole," a conveniently rounded version being six.022 × 1023/mol.
Consequent with its definition as an amount unit of measurement, 1 mole of any element contains the same number of atoms equally one mole of any other chemical element. The masses of ane mole of dissimilar elements, notwithstanding, are different, since the masses of the private atoms are drastically different. The molar mass of an element (or compound) is the mass in grams of 1 mole of that substance, a property expressed in units of grams per mole (yard/mol) (see Figure 4).

Because the definitions of both the mole and the atomic mass unit are based on the same reference substance, 12C, the molar mass of any substance is numerically equivalent to its atomic or formula weight in amu. Per the amu definition, a single 12C atom weighs 12 amu (its atomic mass is 12 amu). Co-ordinate to the definition of the mole, 12 m of 12C contains i mole of 12C atoms (its molar mass is 12 k/mol). This relationship holds for all elements, since their atomic masses are measured relative to that of the amu-reference substance, 12C. Extending this principle, the molar mass of a chemical compound in grams is too numerically equivalent to its formula mass in amu (Figure 5).

| Chemical element | Average Atomic Mass (amu) | Tooth Mass (thou/mol) | Atoms/Mole |
|---|---|---|---|
| C | 12.01 | 12.01 | 6.022 × 1023 |
| H | 1.008 | one.008 | 6.022 × 1023 |
| O | 16.00 | 16.00 | 6.022 × 1023 |
| Na | 22.99 | 22.99 | 6.022 × 1023 |
| Cl | 35.45 | 33.45 | 6.022 × ten23 |
| Table ane. | |||
While atomic mass and molar mass are numerically equivalent, keep in mind that they are vastly different in terms of calibration, as represented by the vast difference in the magnitudes of their corresponding units (amu versus g). To capeesh the enormity of the mole, consider a modest driblet of water weighing virtually 0.03 one thousand (run into Figure 6). Although this represents but a tiny fraction of ane mole of water (~18 g), information technology contains more h2o molecules than tin be clearly imagined. If the molecules were distributed as among the roughly seven billion people on world, each person would receive more than 100 billion molecules.


The mole is used in chemistry to represent half-dozen.022 × 1023 of something, but it tin can be hard to anticipate such a large number. Picket this video then complete the "Retrieve" questions that follow. Explore more almost the mole by reviewing the information under "Dig Deeper."
The relationships between formula mass, the mole, and Avogadro's number can be applied to compute diverse quantities that describe the composition of substances and compounds. For example, if we know the mass and chemical composition of a substance, we tin determine the number of moles and calculate number of atoms or molecules in the sample. Besides, if we know the number of moles of a substance, nosotros can derive the number of atoms or molecules and calculate the substance's mass.
Example 3
Deriving Moles from Grams for an Element
According to nutritional guidelines from the United states of america Department of Agronomics, the estimated average requirement for dietary potassium is 4.7 g. What is the estimated average requirement of potassium in moles?
Solution
The mass of K is provided, and the respective amount of K in moles is requested. Referring to the periodic table, the atomic mass of K is 39.10 amu, and so its molar mass is 39.ten g/mol. The given mass of K (4.7 chiliad) is a fleck more than than 1-tenth the molar mass (39.10 g), so a reasonable "ballpark" estimate of the number of moles would be slightly greater than 0.1 mol.
The tooth amount of a substance may be calculated past dividing its mass (thou) by its tooth mass (g/mol):

The gene-label method supports this mathematical approach since the unit of measurement "g" cancels and the answer has units of "mol:"
[latex]iv.seven \rule[0.5ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{g} \;\text{K} (\frac{\text{mol K}}{39.10 \rule[0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{1000}}) = 0.12 \;\text{mol M}[/latex]
The calculated magnitude (0.12 mol One thousand) is consequent with our ballpark expectation, since it is a fleck greater than 0.1 mol.
Cheque Your Learning
Beryllium is a light metal used to fabricate transparent Ten-ray windows for medical imaging instruments. How many moles of Be are in a thin-foil window weighing 3.24 g?
Example iv
Deriving Grams from Moles for an Element
A liter of air contains 9.ii × 10−4 mol argon. What is the mass of Ar in a liter of air?
Solution
The molar amount of Ar is provided and must be used to derive the corresponding mass in grams. Since the amount of Ar is less than 1 mole, the mass will be less than the mass of 1 mole of Ar, approximately 40 m. The molar amount in question is approximately ane-one thousandth (~10−3) of a mole, and and so the corresponding mass should be roughly one-i thousandth of the molar mass (~0.04 g):

In this example, logic dictates (and the factor-label method supports) multiplying the provided amount (mol) by the molar mass (grand/mol):
[latex]nine.two \times 10^{-iv} \;\rule[0.5ex]{i.75em}{0.1ex}\hspace{-1.75em}\text{mol} \;\text{Ar} (\frac{39.95 \;\text{1000}}{\rule[0.25ex]{1.25em}{0.1ex}\hspace{-1.25em}\text{mol} \;\text{Ar}}) = 0.037 \;\text{g Ar}[/latex]
The result is in agreement with our expectations, around 0.04 g Ar.
Bank check Your Learning
What is the mass of 2.561 mol of gilt?
Case 5
Deriving Number of Atoms from Mass for an Element
Copper is commonly used to fabricate electric wire (Figure seven). How many copper atoms are in 5.00 thou of copper wire?

Solution
The number of Cu atoms in the wire may be conveniently derived from its mass by a two-pace ciphering: first calculating the molar amount of Cu, and so using Avogadro's number (NorthwardA ) to convert this molar amount to number of Cu atoms:

Considering that the provided sample mass (5.00 g) is a little less than 1-10th the mass of 1 mole of Cu (~64 g), a reasonable estimate for the number of atoms in the sample would be on the society of 1-tenth NA , or approximately 1022 Cu atoms. Carrying out the two-step computation yields:
[latex]5.00 \;\dominion[0.5ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{g} \;\text{Cu} (\frac{\rule[0.25ex]{one.25em}{0.1ex}\hspace{-1.25em}\text{mol} \;\text{Cu}}{63.55 \rule[0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{g}})(\frac{6.022 \times 10^{23} \;\text{atoms}}{\rule[0.25ex]{1.25em}{0.1ex}\hspace{-one.25em}\text{mol}}) = 4.74 \times 10^{22} \;\text{atoms of copper}[/latex]
The factor-characterization method yields the desired cancellation of units, and the computed result is on the order of 1022 as expected.
Bank check Your Learning
A prospector panning for gold in a river collects fifteen.00 g of pure gold. How many Au atoms are in this quantity of gold?
Answer:
4.586 × 1022 Au atoms
Case 6
Deriving Moles from Grams for a Compound
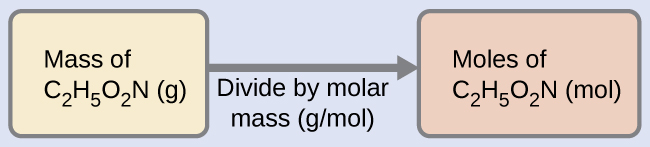
Our bodies synthesize protein from amino acids. One of these amino acids is glycine, which has the molecular formula C2H5O2N. How many moles of glycine molecules are contained in 28.35 g of glycine?
Solution
We tin derive the number of moles of a chemical compound from its mass following the same procedure nosotros used for an chemical element in Example 3:
The tooth mass of glycine is required for this adding, and it is computed in the same fashion as its molecular mass. One mole of glycine, C2H5O2N, contains 2 moles of carbon, v moles of hydrogen, 2 moles of oxygen, and one mole of nitrogen:
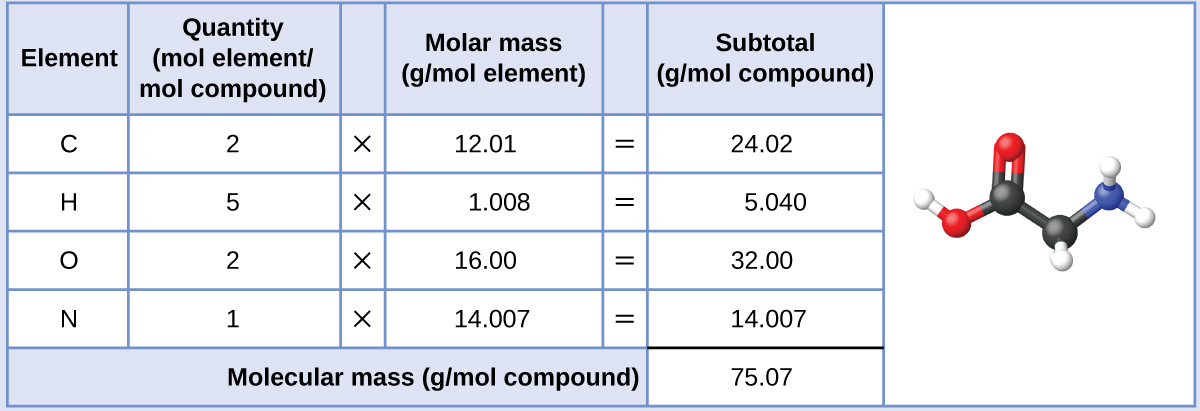
The provided mass of glycine (~28 1000) is a bit more than 1-third the molar mass (~75 g/mol), so we would expect the computed issue to be a fleck greater than one-third of a mole (~0.33 mol). Dividing the compound'due south mass past its molar mass yields:
[latex]28.35 \;\rule[0.5ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{g} \;\text{glycine} \;(\frac{\text{mol glycine}}{75.07 \;\rule[0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{grand}}) = 0.378 \;\text{mol glycine}[/latex]
This result is consistent with our rough guess.
Check Your Learning
How many moles of sucrose, C12H22Oeleven, are in a 25-g sample of sucrose?
Instance 7
Deriving Grams from Moles for a Chemical compound
Vitamin C is a covalent compound with the molecular formula C6H8O6. The recommended daily dietary allowance of vitamin C for children aged 4–8 years is 1.42 × 10−four mol. What is the mass of this allowance in grams?
Solution
Every bit for elements, the mass of a compound can be derived from its molar amount every bit shown:

The molar mass for this chemical compound is computed to be 176.124 g/mol. The given number of moles is a very small fraction of a mole (~10−iv or one-ten thousandth); therefore, nosotros would expect the corresponding mass to be near one-ten thousandth of the molar mass (~0.02 thou). Performing the calculation, we get:
[latex]1.42 \times x^{-4} \;\rule[0.5ex]{1.75em}{0.1ex}\hspace{-i.75em}\text{mol} \;\text{vitamin C} (\frac{176.124 \text{g}}{\rule[0.25ex]{1.25em}{0.1ex}\hspace{-i.25em}\text{mol} \;\text{vitamin C}}) = 0.0250 \;\text{g vitamin C}[/latex]
This is consistent with the anticipated consequence.
Cheque Your Learning
What is the mass of 0.443 mol of hydrazine, NorthwardtwoH4?
Example 8
Deriving the Number of Atoms and Molecules from the Mass of a Chemical compound
A packet of an bogus sweetener contains 40.0 mg of saccharin (C7H5NO3Due south), which has the structural formula:
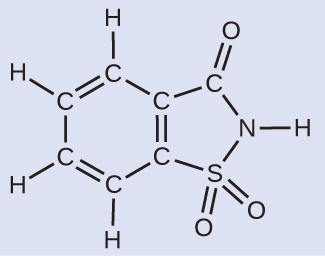
Given that saccharin has a molar mass of 183.18 g/mol, how many saccharin molecules are in a forty.0-mg (0.0400-g) sample of saccharin? How many carbon atoms are in the same sample?
Solution
The number of molecules in a given mass of compound is computed by first deriving the number of moles, as demonstrated in Example half dozen, and then multiplying by Avogadro'south number:
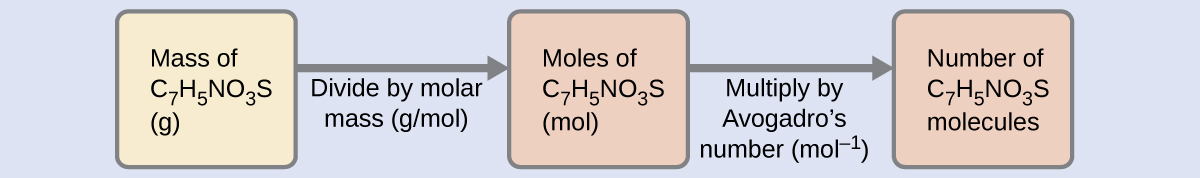
Using the provided mass and molar mass for saccharin yields:
[latex]0.0400 \;\dominion[0.5ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{g} \;\text{C}_7\text{H}_5\text{NO}_3\text{Due south} (\frac{\dominion[0.25ex]{1.25em}{0.1ex}\hspace{-ane.25em}\text{mol} \;\text{C}_7\text{H}_5\text{NO}_3\text{S}}{183.eighteen \;\rule[0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{1000} \;\text{C}_7\text{H}_5\text{NO}_3\text{South}}) (\frac{6.022 \times 10^{23} \;\text{C}_7\text{H}_5\text{NO}_3\text{Southward} \;\text{molecules}}{1\;\rule[0.25ex]{1.25em}{0.1ex}\hspace{-i.25em}\text{mol} \;\text{C}_7\text{H}_5\text{NO}_3\text{Southward}})[/latex] [latex]= one.31 \times 10^{xx} \;\text{C}_7\text{H}_5\text{NO}_3\text{Due south} \;\text{molecules}[/latex]
The compound'south formula shows that each molecule contains 7 carbon atoms, and so the number of C atoms in the provided sample is:
[latex]one.31 \times x^{xx} \;\text{C}_7\text{H}_5\text{NO}_3\text{S molecules} \;(\frac{7 \;\text{C atoms}}{1 \;\text{C}_7\text{H}_5\text{NO}_3\text{S molecule}}) = 9.twenty \times 10^{21} \;\text{C atoms}[/latex]
Check Your Learning
How many C4H10 molecules are independent in ix.213 g of this chemical compound? How many hydrogen atoms?
Answer:
9.545 × 1022 molecules C4 Hten; ix.545 × 1023 atoms H
Counting Neurotransmitter Molecules in the Encephalon
The encephalon is the command center of the central nervous system (Effigy 8). It sends and receives signals to and from muscles and other internal organs to monitor and command their functions; information technology processes stimuli detected by sensory organs to guide interactions with the external world; and information technology houses the complex physiological processes that give rise to our intellect and emotions. The broad field of neuroscience spans all aspects of the construction and function of the central nervous system, including enquiry on the anatomy and physiology of the brain. Dandy progress has been made in brain research over the by few decades, and the Encephalon Initiative, a federal initiative announced in 2013, aims to accelerate and capitalize on these advances through the concerted efforts of various industrial, bookish, and government agencies (more details available at www.whitehouse.gov/share/encephalon-initiative).
Specialized cells chosen neurons transmit data between different parts of the fundamental nervous system by way of electric and chemical signals. Chemical signaling occurs at the interface between different neurons when 1 of the cells releases molecules (called neurotransmitters) that diffuse across the pocket-sized gap between the cells (called the synapse) and bind to the surface of the other jail cell. These neurotransmitter molecules are stored in pocket-sized intracellular structures chosen vesicles that fuse to the cell wall and and then suspension open to release their contents when the neuron is appropriately stimulated. This process is called exocytosis (see Figure 9). One neurotransmitter that has been very extensively studied is dopamine, C8H11NOtwo. Dopamine is involved in various neurological processes that impact a wide variety of human behaviors. Dysfunctions in the dopamine systems of the brain underlie serious neurological diseases such as Parkinson's and schizophrenia.
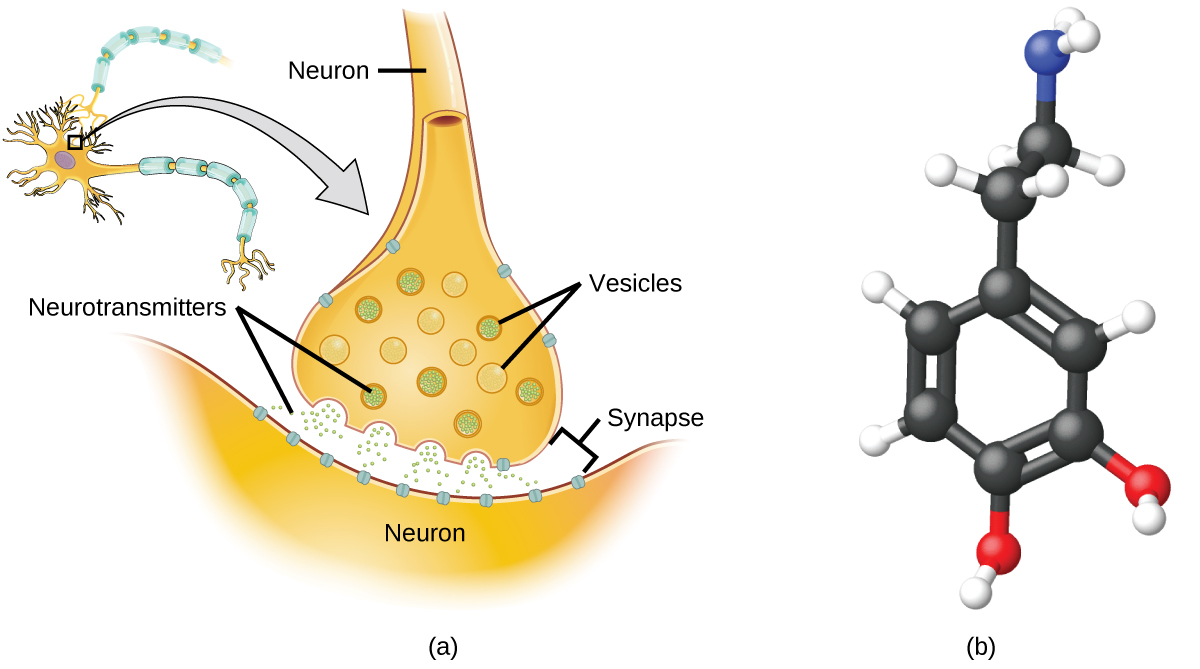
Ane of import aspect of the complex processes related to dopamine signaling is the number of neurotransmitter molecules released during exocytosis. Since this number is a central gene in determining neurological response (and subsequent human thought and action), it is important to know how this number changes with certain controlled stimulations, such every bit the administration of drugs. It is besides of import to understand the mechanism responsible for any changes in the number of neurotransmitter molecules released—for instance, some dysfunction in exocytosis, a change in the number of vesicles in the neuron, or a change in the number of neurotransmitter molecules in each vesicle.
Significant progress has been fabricated recently in directly measuring the number of dopamine molecules stored in private vesicles and the amount actually released when the vesicle undergoes exocytosis. Using miniaturized probes that tin selectively detect dopamine molecules in very small amounts, scientists have determined that the vesicles of a sure type of mouse encephalon neuron contain an boilerplate of xxx,000 dopamine molecules per vesicle (about 5 × 10−20 mol or fifty zmol). Analysis of these neurons from mice subjected to various drug therapies shows significant changes in the average number of dopamine molecules contained in private vesicles, increasing or decreasing by up to three-fold, depending on the specific drug used. These studies also indicate that not all of the dopamine in a given vesicle is released during exocytosis, suggesting that it may be possible to regulate the fraction released using pharmaceutical therapies.[1]
Key Concepts and Summary
The formula mass of a substance is the sum of the boilerplate atomic masses of each atom represented in the chemical formula and is expressed in diminutive mass units. The formula mass of a covalent chemical compound is too called the molecular mass. A convenient amount unit of measurement for expressing very large numbers of atoms or molecules is the mole. Experimental measurements have adamant the number of entities composing 1 mole of substance to be half dozen.022 × 1023, a quantity called Avogadro's number. The mass in grams of 1 mole of substance is its molar mass. Due to the use of the same reference substance in defining the diminutive mass unit and the mole, the formula mass (amu) and molar mass (g/mol) for any substance are numerically equivalent (for instance, one HtwoO molecule weighs approximately xviii amu and i mole of HtwoO molecules weighs approximately 18 g).
Chemistry End of Chapter Exercises
- What is the total mass (amu) of carbon in each of the following molecules?
(a) CH4
(b) CHClthree
(c) C12H10O6
(d) CH3CHiiCH2CH2CHiii
- What is the full mass of hydrogen in each of the molecules?
(a) CH4
(b) CHCl3
(c) C12H10O6
(d) CH3CH2CH2CH2CHiii
- Calculate the molecular or formula mass of each of the post-obit:
(a) Pfour
(b) H2O
(c) Ca(NO3)2
(d) CHiiiCO2H (acetic acrid)
(e) C12H22O11 (sucrose, cane sugar).
- Decide the molecular mass of the post-obit compounds:
(a)
(b)
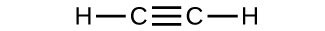
(c)
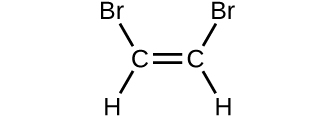
(d)
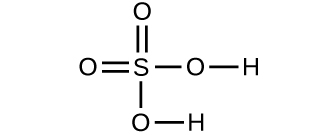
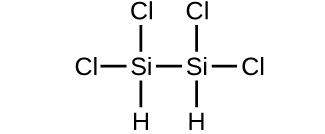
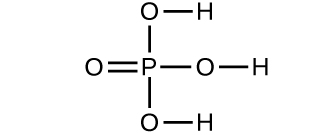
- Determine the molecular mass of the following compounds:
(a)
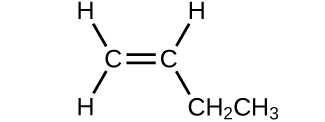
(b)
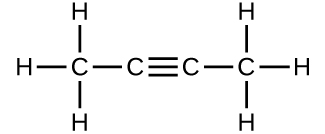
(c)
(d)
- Which molecule has a molecular mass of 28.05 amu?
(a)
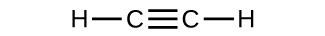
(b)
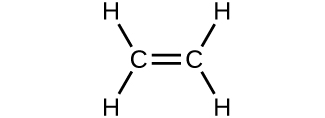
(c)
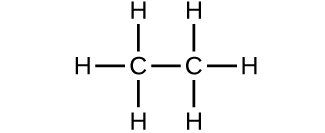
- Write a sentence that describes how to determine the number of moles of a compound in a known mass of the compound if we know its molecular formula.
- Compare ane mole of Htwo, 1 mole of O2, and i mole of Fii.
(a) Which has the largest number of molecules? Explain why.
(b) Which has the greatest mass? Explain why.
- Which contains the greatest mass of oxygen: 0.75 mol of ethanol (CiiHfiveOH), 0.threescore mol of formic acid (HCO2H), or one.0 mol of water (H2O)? Explain why.
- Which contains the greatest number of moles of oxygen atoms: 1 mol of ethanol (CiiHvOH), ane mol of formic acid (HCO2H), or 1 mol of water (H2O)? Explicate why.
- How are the molecular mass and the molar mass of a compound similar and how are they different?
- Calculate the molar mass of each of the post-obit compounds:
(a) hydrogen fluoride, HF
(b) ammonia, NH3
(c) nitric acid, HNOthree
(d) silver sulfate, Ag2And then4
(e) boric acid, B(OH)3
- Summate the molar mass of each of the post-obit:
(a) S8
(b) C5H12
(c) Scii(Then4)3
(d) CH3COCHiii (acetone)
(e) C6H12O6 (glucose)
- Calculate the empirical or molecular formula mass and the molar mass of each of the following minerals:
(a) limestone, CaCOiii
(b) halite, NaCl
(c) beryl, Be3AliiSihalf dozenO18
(d) malachite, Cutwo(OH)2COthree
(east) turquoise, CuAlhalf dozen(POfour)4(OH)8(H2O)4
- Calculate the molar mass of each of the following:
(a) the anesthetic halothane, C2HBrClF3
(b) the herbicide paraquat, C12H14NiiClii
(c) caffeine, CeightH10NfourOtwo
(d) urea, CO(NH2)2
(e) a typical soap, C17H35COiiNa
- Determine the number of moles of compound and the number of moles of each type of atom in each of the following:
(a) 25.0 g of propylene, CthreeHvi
(b) 3.06 × 10−3 yard of the amino acrid glycine, C2H5NOii
(c) 25 lb of the herbicide Treflan, C13H16NtwoO4F (1 lb = 454 g)
(d) 0.125 kg of the insecticide Paris Green, Cu4(AsO3)two(CH3CO2)2
(east) 325 mg of aspirin, Chalf-dozenH4(COiiH)(CO2CH3)
- Decide the mass of each of the following:
(a) 0.0146 mol KOH
(b) x.2 mol ethane, C2H6
(c) i.half-dozen × 10−three mol Na2 SO4
(d) 6.854 × 103 mol glucose, Cvi H12 Ohalf-dozen
(eastward) 2.86 mol Co(NH3)6Cliii
- Make up one's mind the number of moles of the compound and determine the number of moles of each type of atom in each of the following:
(a) 2.12 g of potassium bromide, KBr
(b) 0.1488 thousand of phosphoric acrid, H3POiv
(c) 23 kg of calcium carbonate, CaCOthree
(d) 78.452 k of aluminum sulfate, Altwo(And so4)iii
(east) 0.1250 mg of caffeine, C8HxNorth4O2
- Decide the mass of each of the following:
(a) 2.345 mol LiCl
(b) 0.0872 mol acetylene, CtwoH2
(c) 3.iii × x−2 mol Na2 COthree
(d) 1.23 × 103 mol fructose, Chalf-dozen H12 Osix
(e) 0.5758 mol FeSOfour(H2O)7
- The approximate minimum daily dietary requirement of the amino acrid leucine, CsixHxiiiNO2, is ane.1 g. What is this requirement in moles?
- Determine the mass in grams of each of the following:
(a) 0.600 mol of oxygen atoms
(b) 0.600 mol of oxygen molecules, O2
(c) 0.600 mol of ozone molecules, Othree
- A 55-kg woman has 7.5 × 10−3 mol of hemoglobin (molar mass = 64,456 yard/mol) in her claret. How many hemoglobin molecules is this? What is this quantity in grams?
- Make up one's mind the number of atoms and the mass of zirconium, silicon, and oxygen found in 0.3384 mol of zircon, ZrSiO4, a semiprecious stone.
- Make up one's mind which of the post-obit contains the greatest mass of hydrogen: 1 mol of CHiv, 0.six mol of C6Hhalf dozen, or 0.4 mol of CthreeH8.
- Decide which of the post-obit contains the greatest mass of aluminum: 122 thou of AlPOfour, 266 g of AltwoC16, or 225 m of Al2Due south3.
- Diamond is ane grade of elemental carbon. An engagement ring contains a diamond weighing ane.25 carats (ane carat = 200 mg). How many atoms are present in the diamond?
- The Cullinan diamond was the largest natural diamond ever found (January 25, 1905). It weighed 3104 carats (1 carat = 200 mg). How many carbon atoms were present in the stone?
- One 55-gram serving of a detail cereal supplies 270 mg of sodium, 11% of the recommended daily assart. How many moles and atoms of sodium are in the recommended daily allowance?
- A certain nut crunch cereal contains 11.0 grams of saccharide (sucrose, C12H22O11) per serving size of 60.0 grams. How many servings of this cereal must be eaten to consume 0.0278 moles of sugar?
- A tube of toothpaste contains 0.76 m of sodium monofluorophosphate (NatwoPO3F) in 100 mL.
(a) What mass of fluorine atoms in mg was present?
(b) How many fluorine atoms were nowadays?
- Which of the following represents the least number of molecules?
(a) 20.0 1000 of HtwoO (18.02 g/mol)
(b) 77.0 g of CH4 (16.06 yard/mol)
(c) 68.0 g of CaH2 (42.09 g/mol)
(d) 100.0 chiliad of Due north2O (44.02 yard/mol)
(e) 84.0 g of HF (20.01 g/mol)
Glossary
- Avogadro's number (Due northA )
- experimentally determined value of the number of entities comprising 1 mole of substance, equal to six.022 × 1023 mol−1
- formula mass
- sum of the average masses for all atoms represented in a chemical formula; for covalent compounds, this is also the molecular mass
- molar mass
- mass in grams of i mole of a substance
- mole
- amount of substance containing the same number of atoms, molecules, ions, or other entities every bit the number of atoms in exactly 12 grams of 12C
Solutions
Answers to Chemistry End of Chapter Exercises
1. (a) 12.01 amu; (b) 12.01 amu; (c) 144.12 amu; (d) threescore.05 amu
3. (a) 123.896 amu; (b) 18.015 amu; (c) 164.086 amu; (d) 60.052 amu; (due east) 342.297 amu
5. (a) 56.107 amu; (b) 54.091 amu; (c) 199.9976 amu; (d) 97.9950 amu
vii. Apply the molecular formula to find the tooth mass; to obtain the number of moles, split up the mass of compound by the molar mass of the compound expressed in grams.
nine. Formic acid. Its formula has twice as many oxygen atoms as the other two compounds (1 each). Therefore, 0.60 mol of formic acid would be equivalent to i.20 mol of a compound containing a unmarried oxygen atom.
xi. The two masses have the same numerical value, simply the units are different: The molecular mass is the mass of 1 molecule while the molar mass is the mass of half dozen.022 × ten23 molecules.
13. (a) 256.528 g/mol; (b) 72.150 g mol−1; (c) 378.103 one thousand mol−1; (d) 58.080 yard mol−1; (e) 180.158 g mol−1
15. (a) 197.382 g mol−1; (b) 257.163 g mol−i; (c) 194.193 thou mol−i; (d) lx.056 yard mol−i; (east) 306.464 grand mol−ane
17. (a) 0.819 one thousand; (b) 307 g; (c) 0.23 1000; (d) ane.235 × tenvi g (1235 kg); (e) 765 m
xix. (a) 99.41; (b) two.27 grand; (c) 3.5 m; (d) 222 kg; (e) 160.1 thou
21. (a) ix.60 k; (b) 19.ii k; (c) 28.8 k
23. zirconium: 2.038 × 1023 atoms; 30.87 grand; silicon: 2.038 × ten23 atoms; ix.504 g; oxygen: 8.151 × ten23 atoms; 21.66 thou
25. AlPO4: 1.000 mol
Al2Clvi: 1.994 mol
AltwoS3: 3.00 mol
27. 3.113 × 1025 C atoms
29. 0.865 servings, or about ane serving.
31. 20.0 thou H2O represents the to the lowest degree number of molecules since information technology has the least number of moles.
whitfeldtuaid1940.blogspot.com
Source: https://opentextbc.ca/chemistry/chapter/3-1-formula-mass-and-the-mole-concept/

0 Response to "If You Know the Grams of Formula How Do You Find Moles"
Post a Comment